A Retrospective Study to Investigate and Evaluate the Unanticipated Results of Hair Loss Cessation and Temporary Hair Regrowth in Adult Males Who Had Undergone Low Level Laser Therapy for Hair Wellness.
Author:
Glenn Charles, DO
Physician Researcher for Hair Solutions of New York
Introduction: This brief study is a direct by-product of a program originally designed to improve cosmetic hair wellness and hair quality in individuals using a low-level laser therapy (LLLT) device. For the purpose of this study, hair wellness and hair quality are being defined by increased thickness and density, suppleness, sheen, silkiness and color treatment retention. The device being evaluated is an Aluminum, Gallium, Indium, Phosphorous (AlGaInP) low level laser known as the Laser Hair Care 4000 device. It is classified as a class IIIa laser device for cosmetic use.
As the project continued and the subjects enrolled began receiving the normal course of treatments administered for hair wellness, technicians and clients alike observed the unanticipated effects of hair loss cessation and demonstrable hair regrowth. Such observations lead to postulate that further study was indicated. With that in mind, a prospective, randomized, double blinded, multi-center clinical trial is being developed to determine if the Laser Hair Care system can lead to hair growth in the same way minoxidil and finesteride are currently being used for the treatment of androgenetic alopecia. The Food and Drug Administration has approved the latter for this indication.
All subjects received treatment in a single center for 16 weeks, under the care of the clinic’s staff. Subjects in this study presented themselves to the clinic hoping to improve their hair quality. Although there were more than 300 total subjects enrolled for the hair wellness therapy, only 25 had adequate documentation for performing accurate hair counts at baseline and at the completion of the 16 weeks.
Purpose: To evaluate the available data on the safety and efficacy of a low level laser device in its possible application as a therapy for treating patients experiencing androgenetic alopecia.
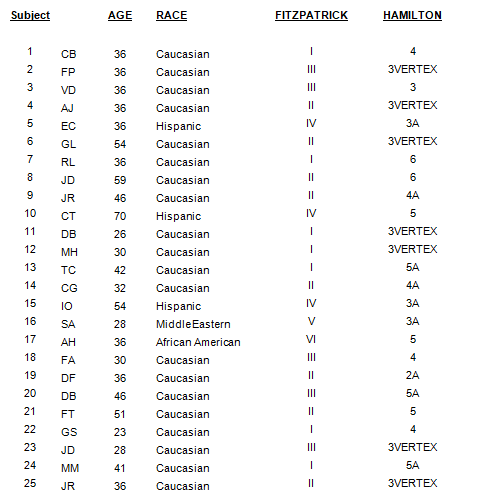
Materials & Methods: Complete and reliable data was collected from 25 adult, male subjects between the ages 23 and 50, having Hamilton-Norwood classifications between stages II and V and Fitzpatrick skin types 1 through 5 were treated using the Laser device. The laser device includes 30 continuous wave diodes at 670nm. The subjects received two 15-minute treatments per week, for their initial 5-week protocol, decreasing to one 15-minute treatment per week for the remainder of their program.
Hair counts were performed at the beginning and end of each subjects’ respective date range. Each subject had hair counts performed on 3 different scalp locations: vertex, temporal and frontal area. Counts were obtained using a Capilliscope camera (a digital diagnostic camera using a 50X magnification) and proprietary, densitometry counting software. Baseline hair counts performed were intended primarily to assess success in improving the quality of hair in the treatment region, throughout the course of treatment program. Similarly, all hair counts were performed at the conclusion of the treatment period to track improvements in the subjects’ overall hair wellness and quality. Unanticipated improvement in hair counts observed were recorded as well.
A technician who had undergone training and certification by the manufacturer of the Capilliscope camera, exclusively performed all hair counts. The technician was trained in the setup, and management of the camera and densitometry software. The counts were made in an area that is one square centimeter, then digitally archived for comparative analysis.
Results: The table below summarizes the combined results for all subjects. Significant hair growth was observed in each area where the counts were performed. The overall average growth for all subjects was 37.79%.
Adverse Reactions: All subjects were questioned about any untoward effects from the therapy. Specifically, each subject was questioned on the presence of headache, pruritus, erythema, burning and tingling sensations. There were no headaches, pruritus, erythema, burning or tingling sensations observed by the interviewer or reported by any subjects.
Data Analysis:
Growth rates reflect the average change of hair counts from baseline date for subjects as measured in 3 scalp locations.
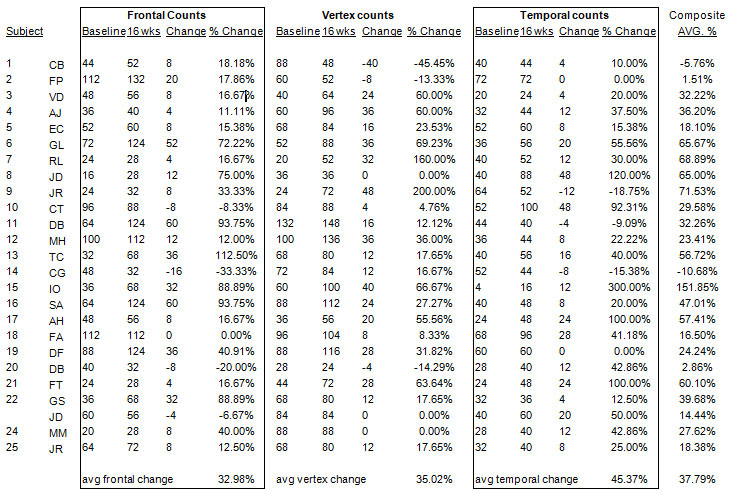
Conclusions: The AlGaInP Laser Hair Care device has demonstrated efficacy for the cessation of hair loss and stimulation of hair growth. In addition, the anecdotal data summarized herein indicates that a multi-center, randomized, double blinded, clinical trial exploring the possible application of the Laser Hair Care device in these areas is warranted.
Demographic Analysis: The following data is presented as study participant background data. These categories was used to further assess the quality and quantity of improvement observed.